
ABOUT COLUMBUS
The COLUMBUS trial was a randomised, Phase 3, multicentre, open-label, active-controlled study3,4,5,7,11
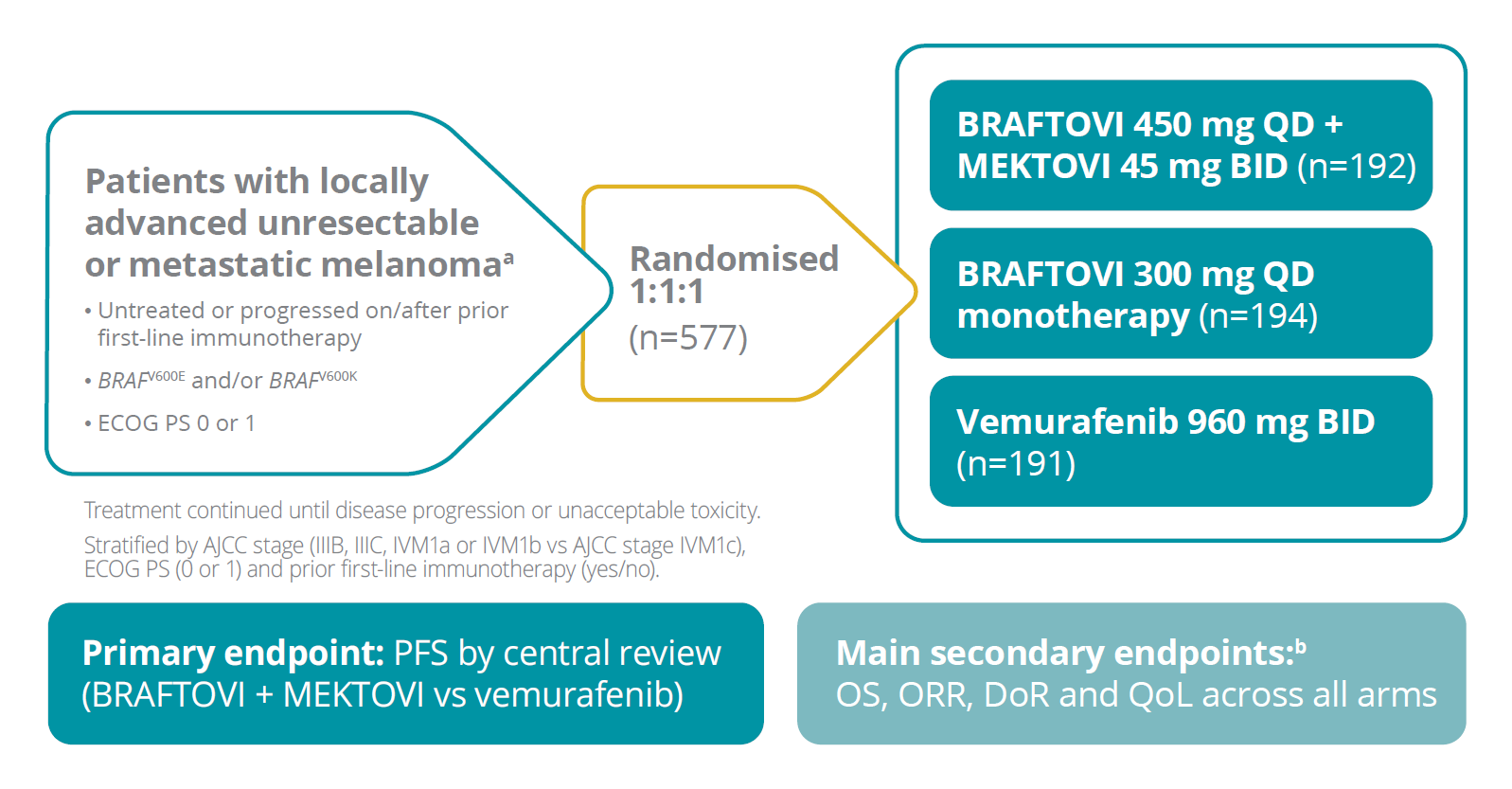
AJCC, American Joint Committee on Cancer; BID, twice daily; DoR, duration of response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; QD, once daily; QoL, quality of life.
aFor a full list of inclusion and exclusion criteria, please refer to the Summaries of Product Characteristics.1,2 bAll secondary endpoints are descriptive in nature.
Explore COLUMBUS efficacy results on the efficacy page
BRAFTOVI® + MEKTOVI® was studied in a broad range of patients11,12
95% of patients in COLUMBUS were treatment naïve for metastatic disease4
| Select baseline characteristics4 | BRAFTOVI® (450 mg) + MEKTOVI® (45 mg)a n=192 |
vemurafenib (960 mg) n=191 |
| Median age (range), years | 57 (20–89) | 56 (21–82) |
| ECOG PS 0, % | 71 | 73 |
| LDH ≥ULN, % | 29 | 27 |
| BRAFV600E/K mutation status, % | 89/11 | 88/12 |
| Tumour stage at study entry, % | ||
| IIIB/IIIC | 5 | 6 |
| IVM1a | 14 | 13 |
| IVM1b | 18 | 16 |
| IVM1c | 64 | 65 |
| Number of organs involved, % | ||
| 1 | 24 | 24 |
| 2 | 30 | 31 |
| ≥3 | 45 | 46 |
| Previous immunotherapy in advanced or metastatic setting, % | ||
| Ipilimumab | 3 | 3 |
| Anti-PD1 or anti-PD-L1 | 1 | 0 |
| Interferons or interleukins | 2 | 3 |
BID, twice daily; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LDH, lactate dehydrogenase; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; QD, once daily; ULN, upper limit of normal. aDosing regimen for the combination was BRAFTOVI 450 mg QD + MEKTOVI 45 mg BID and BRAFTOVI 300 mg QD.4


